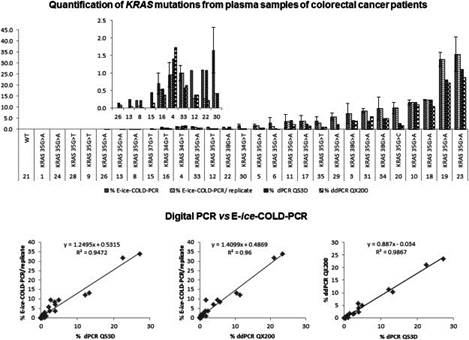
Circulating cell-free DNA (ccfDNA) bears great promise as biomarker for personalized medicine, but ccfDNA is present only at low levels in the plasma or serum of cancer patients. E-ice-COLD-PCR is a recently developed enrichment method to detect and identify mutations present at low-abundance in clinical samples. However, recent studies have shown the importance to accurately quantify low-abundance mutations as clinically important decisions will depend on certain mutation thresholds. The possibility for an enrichment method to accurately quantify the mutation levels remains a point of concern and might limit its clinical applicability. David S. et al. compared the quantification ofKRASmutations in ccfDNA from metastatic colorectal cancer patients by E-ice-COLD-PCR with two digital PCR approaches. For the quantification of mutations by E-ice-COLD-PCR, cell lines with known mutations diluted into WT genomic DNA were used for calibration. E-ice-COLD-PCR and the two digital PCR approaches showed the same range of the mutation level and were concordant for mutation levels below the clinical relevant threshold. E-ice-COLD-PCR can accurately detect and quantify low-abundant mutations in ccfDNA and has a shorter time to results making it compatible with the requirements of analyses in a clinical setting without the loss of quantitative accuracy.
Sefrioui D, Mauger F, Leclere L, et al.Clinica Chimica Acta, 2017, 465:1-4.













